
Comprehensive analysis of allulose production: review and update
Allulose StoreSource: mdpi.com
August 17, 2024
1. Department of Cereal Science and Technology, Jiangsu University of Science and Technology, Zhenjiang 212100, China
2. Jiangsu Provincial Grain Bioprocessing Engineering Research Center, Zhenjiang 212100, China
3. Department of Computer Science, Jiangsu University of Science and Technology, Zhenjiang 212100, China
In recent years, significant advances have been made in the production of D-allulose, especially with regard to enzymatic conversion methods.
Key developments include traditional immobilization techniques, discovery of new enzymes, directed evolution studies, and biosynthesis through modification of metabolic pathways.
Enzymatic conversion, especially using D-allulose-3-epimerase, remains essential for industrial-scale production.
Innovative immobilization strategies, such as functionalized nano-beads and magnetic MOF nanoparticles, have significantly improved the stability and reusability of enzymes.
Directed evolution has led to improvements in the thermostability and catalytic efficiency of enzymes, while synthetic biology methods, including phosphorylation-driven and thermodynamically driven pathways, have optimized production processes.
High-throughput screening methods have been key in identifying and refining enzyme variants for industrial applications.
Together, these developments not only increase production efficiency and cost-effectiveness, but also align with sustainable and economically viable manufacturing practices.
The past five years have witnessed critical developments that could have a significant potential impact on the commercial viability and global demand for allulose.
1. Introduction
Allulose, also known as psicose, is a rare sugar that has gained significant interest worldwide due to its low calorie content and potential health benefits, particularly in the treatment of diabetes and obesity.
Increasing consumer awareness of healthy eating habits has significantly increased the global demand for allulose [ 1 , 2 , 3 , 4 ].
North America, especially the United States, is a leader in production and technological developments, with companies such as Ingredion and Tate & Lyle.
The proliferation of allulose in various food and beverage products has been strong, further accelerating demand [ 5 ].
In addition to the United States, Japan and South Korea are also prominent markets, with companies such as Matsutani Chemical and CJ CheilJedang pioneering the production and application of allulose [ 6 ].
The production of allulose requires sophisticated technologies, primarily enzymatic conversion and synthetic biology [ 7 , 8 , 9 , 10 ].
Enzymatic conversion , which uses specific enzymes to convert fructose to allulose, is widely preferred due to its high yield and efficiency.
The enzyme D-allulose-3-epimerase is involved in the conversion of fructose to allulose, which changes the configuration of the hydroxyl group on the third carbon atom of fructose, resulting in allulose ( Figure 1 ) [ 11 , 12 ].
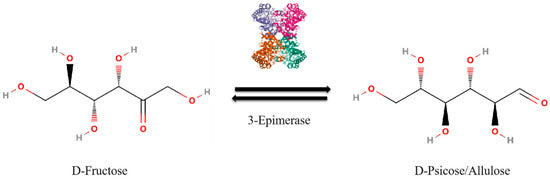
The rate of conversion of fructose to allulose remains around 30% due to thermodynamic equilibrium.
In the enzymatic reaction catalyzed by D-allulose-3-epimerase, the forward (fructose to allulose) and reverse (allulose to fructose) reactions reach equilibrium and stabilize at this ratio [ 13 , 14 , 15 , 16 , 17 , 18 ].
This equilibrium reflects the free energy landscape of the reaction, where the energy difference between fructose and allulose is minimal. Thus, at equilibrium, the forward and reverse reaction rates are equal, limiting further conversion [ 14 , 19 , 20 , 21 , 22 , 23 ].
To overcome this obstacle, innovative approaches are needed to shift the balance towards higher allulose yields.
Synthetic biology offers a promising solution to this balance barrier. By genetically modifying microorganisms to optimize metabolic pathways, the efficiency of the transformation process can be increased.
This approach can shift the equilibrium towards higher yields of allulose, overcoming natural thermodynamic limits [ 8 , 24 , 25 , 26 ].

- Figure 1. Schematic representation of the reaction where D-fructose is converted to D-allulose in the presence of 3-epimerase.
Significant progress has been made in the regulatory environment for allulose.
In 2019, the United States Food and Drug Administration (FDA) recognized allulose as a generally recognized as safe (GRAS) substance, accelerating its market penetration [ 27 ].
Companies like Ingredion and Tate & Lyle have been granted GRAS status, allowing for widespread distribution in the United States.
The European Food Safety Authority (EFSA) is also evaluating the use of allulose in the European Union, and recent data suggest a positive outcome [ 24 ].
China is becoming an increasingly important player in the allulose market, focusing on reducing sugar consumption and improving public health. Chinese companies are investing heavily in advanced production technologies and expanding their capacities to meet both domestic and international demand.
Baolingbao Biology Co., Ltd. (Yucheng, China) has a production capacity of 5,000 tons per year, while Shandong Sanyuan Biotechnology and CJ CheilJedang (Bingzhou, China) contribute significantly with capacities of 3,500 and 4,000 tons per year, respectively.
These multi-million dollar investments highlight their commitment to meeting the growing demand for allulose.
Regulatory support has been critical in China. The Chinese National Health Commission (NHC) approved allulose as a novel food ingredient in 2020, facilitating its incorporation into a wider range of foods [ 28 ].
This regulatory support, combined with China's growing production capacity, is expected to significantly reduce production costs and contribute more significantly to human health by providing a scarce sugar alternative.
Therefore, it is necessary to review the latest achievements in allulose production technology and research [ 6 , 17 , 29 , 30 , 31 , 32 , 33 ].
2. Latest results
2.1. Enzymatic transformation
D-allulose-3-epimerase should be discussed from four different perspectives: (1) Traditional methods involving immobilization and whole-cell fixed catalysis for large-scale production, (2) discovery of new enzymes, (3) directed evolution studies, and (4) biosynthesis by modification of metabolic pathways. Enzymatic conversion remains a fundamental technology for the production of allulose, especially the isomerization of fructose to allulose using specific enzymes such as D-allulose-3-epimerase.
Due to the gradual expansion of market demand, the identification of new 3-epimerase enzymes and the application of immobilization and synthetic biology methods are critical strategies for the development of current industrial processes. Here, we update the research results of the past five years, focusing on optimizing the activity and stability of enzymes to increase conversion efficiency ( Table 1 ) [ 8 , 11 , 34 , 35 ].
2.1.1. Recording
Sodium alginate, as a food-grade immobilization method, is widely used in the current production of allulose, but new methods are constantly emerging that represent significant advances in enzyme stabilization and efficiency [ 36 ].
For example, a study investigating the biotransformation of D-allulose from D-glucose, which included separation by simulated moving bed chromatography (SMBC) and purification by crystallization, demonstrated an impressive 98% pure D-allulose in 70% yield [ 12 ].
This integrated process takes advantage of the high selectivity of SMBC to efficiently separate D-allulose from the reaction mixture, followed by crystallization to achieve high purity.
The ability to achieve such high purity and yield makes this process highly attractive for industrial-scale production, ensuring efficient conversion and separation.
Further innovation is seen in the application of functionalized polyhydroxyalkanoate (PHA) nano-beads as stable biocatalysts.
These nano-beads were designed to immobilize D-allulose-3-epimerase, resulting in enhanced enzyme stability and reusability.
Specifically, the enzyme immobilized on PHA nano-beads retained 85% of its initial activity after 10 cycles of use, compared to the free enzyme, which lost 50% of its activity after only 5 cycles.
This significant improvement in stability and reusability reduces production costs and makes the process more sustainable. The functionalized PHA nano-beads provide a durable platform that minimizes enzyme degradation, allowing for longer-term use in industrial processes.
Furthermore, the two-step biosynthesis of D-allulose through a multienzyme cascade for the bioconversion of fruit juices demonstrated the possibility of combining multiple enzymes in a single process [37].
This method involved the sequential use of fructose isomerase and D-allulose-3-epimerase, which improved the overall efficiency and yield of D-allulose production.
The study reported a 75% conversion rate of D-fructose to D-allulose, with a total yield of 60% from the initial juice substrate.
This efficient conversion process is particularly advantageous when using complex substrates, such as fruit juices, by optimizing each step of the enzymatic conversion.
By using a multienzyme cascade, this approach provides higher overall yields and reduces the need for additional processing steps, thereby simplifying the production process.
Finally, the immobilization of D-allulose-3-epimerase into magnetic metal-organic framework (MOF) nanoparticles represents a cutting-edge approach to enzyme immobilization [ 38 ].
These magnetic MOF nanoparticles allowed for easy recovery and reuse of the enzyme, significantly increasing the efficiency of the biocatalytic process.
The study showed that the immobilized enzyme retained 90% of its activity after 15 cycles, compared to the free enzyme, whose activity dropped to 40% after the same number of cycles.
Additionally, the MOF nanoparticles provided a large surface area for enzyme immobilization, improving the stability and activity of the enzyme.
The magnetic properties of the nanoparticles facilitated their simple separation from the reaction mixture using a magnetic field, making the process extremely practical for large-scale biocatalysis.
This innovative method not only improves the performance of enzymes, but also simplifies further processing, which is critical for industrial applications.
Together, these studies highlight significant advances in immobilization techniques that improve enzyme stability, reusability, and overall efficiency in D-allulose production.
The use of SMBC and crystallization provides high purity and yield, making it a robust method for industrial applications.
Functionalized PHA nano-beads provide a sustainable and cost-effective platform for enzyme immobilization, extending enzyme lifetime and reducing costs.
The multi-enzyme cascade approach optimizes the conversion process, especially for complex substrates, increasing overall production efficiency. Magnetic MOF nanoparticles offer a cutting-edge solution for enzyme recovery and reuse, improving both stability and activity.
Together, these innovative methods pave the way for more sustainable and cost-effective industrial processes, ultimately contributing to the availability of healthier allulose alternatives and advancing the field of industrial biotechnology.
2.1.2. Testing of new industrial enzymes
In order to discover new industrial enzymes, many studies have focused on the characterization and enhancement of D-allulose-3-epimerases, which are key in the production of rare sugars. The recombinant D-allulose-3-epimerase of Agrobacterium sp. ATCC 31749 revealed a critical interfacial amino acid, Lys-152, which is essential for catalytic activity and whose mutations reduce activity by 70%, providing insights into enzyme design [ 39 ].
This finding is crucial as it provides a foundation for understanding how individual amino acid residues contribute to enzyme function, thereby guiding future efforts to design more efficient and stable enzymes for industrial applications.
The putative Dolichol phosphate mannose synthase of Bacillus sp. showed a 35% conversion rate from D-fructose to D-allulose at 50 °C and maintained 80% activity after 24 h [ 40 ].
The dual functionality of this enzyme, which exhibits both D-allulose 3-epimerase activity and the ability to convert D-fructose to D-allulose, could simplify industrial production processes by reducing the need for multiple enzymes. This stability and efficiency are key in industrial applications where continuous production processes are essential, as it offers potential cost savings and simplifies manufacturing workflows.
Another D-allulose-3-epimerase from Bacillus sp. showed remarkable thermal stability, with a half-life of 120 min at 70 °C, converting 30% of D-fructose to D-allulose at an optimal temperature of 65 °C [ 10 ].
The enzyme's ability to function effectively at high temperatures makes it particularly suitable for industrial applications where thermal stability is critical. This property reduces the need for cooling systems, thereby reducing energy costs and increasing the overall efficiency of the production process.
The enzyme's high temperature tolerance also opens up the possibility of its use in processes where maintaining lower temperatures would not be practical or cost-effective.
A novel D-allulose 3-epimerase gene from the metagenome of a thermal aquatic habitat, expressed in Bacillus subtilis, achieved a 40% conversion rate from D-fructose to D-allulose at 60 °C.
The enzyme retained 75% of its activity even after 48 hours of continuous operation, indicating its robustness and suitability for industrial use [ 13 ].
The use of whole-cell catalysis further demonstrates the enzyme's ability for scalable D-allulose production, offering a cost-effective and efficient method for producing this rare sugar.
This approach exploits the natural ability of whole cells to carry out complex biochemical transformations, simplifying the production process and potentially reducing costs associated with enzyme purification and stabilization.
D-tagatose 3-epimerase from Caballeronia fortuita showed broad substrate specificity, converting 25% of D-fructose to D-allulose at 55 °C with an activity of 1.2 U/mg protein [ 41 ].
The ability to produce a variety of rare sugars from different substrates simplifies the production process and reduces costs.
This versatility makes the Caballeronia fortuita enzyme a valuable tool for industries looking to produce high-value sugar alternatives in a cost-effective manner. The enzyme's broad substrate range and efficiency in converting multiple sugars highlight its potential for a wide range of industrial applications, including the production of rare sugars for the food and pharmaceutical industries.
The enzyme from Christensenella minuta showed a conversion rate of 32% at 50 °C with a half-life of 150 min, highlighting its potential for sustainable sugar production [ 42 ].
The enzyme's efficiency and stability at moderate temperatures make it suitable for various industrial applications, including food and beverage manufacturing, where mild processing conditions are preferred to preserve product quality.
The robustness of the enzyme under practical industrial conditions suggests that it can be integrated into existing production systems with minimal modification, increasing the sustainability and efficiency of sugar production processes.
Directed evolution techniques were used to enhance the thermostability of Clostridium cellulolyticum H10 D-allulose 3-epimerase [43].
The improved enzyme increased its half-life from 30 min to 180 min at 65 °C and achieved a 45% conversion rate of D-fructose to D-allulose.
These improvements make the enzyme more viable for industrial applications, where prolonged stability at high temperatures is often required.
The application of directed evolution to improve enzyme properties highlights the potential to tailor enzymes to specific industrial needs, thereby optimizing production processes.
This approach highlights the power of modern biotechnological methods in creating enzymes with enhanced properties tailored for specific industrial applications.
The hyperthermostable l -ribulose-3-epimerase from Labedella endophytica retained 90% of its activity after 24 h at 80 °C, with an optimum temperature of 75 °C [ 21 ].
The enzyme's ability to convert 28% of D-fructose to D-allulose under these conditions underscores its potential for high-temperature industrial processes. Its robustness makes it a promising candidate for applications where extreme conditions exist, such as in the production of certain chemicals and pharmaceuticals. The enzyme's high thermostability means that it can also be used in processes that require sustained high temperatures, potentially reducing the need for cooling and stabilization steps, thereby reducing overall production costs.
The Novibacillus thermophilus enzyme showed optimal activity at 70 °C and retained 85% of its activity at this temperature after 12 h [ 44 ].
It achieved a 38% conversion of D-fructose to D-allulose, demonstrating its efficiency in industrial-scale sugar synthesis. The enzyme's efficiency and thermal stability make it particularly advantageous for the production of D-allulose in processes that require prolonged enzyme activity.
A food-grade expression system in Corynebacterium glutamicum facilitated the conversion of cane molasses to D-allulose with a conversion efficiency of 42%, retaining 80% of its activity after 24 h [ 36 ].
This system enhances the production and application of the enzyme in the food industry, providing a cost-effective and scalable method for producing D-allulose from readily available raw materials such as molasses. This innovation has significant implications for the food industry, where there is a growing demand for healthier allulose alternatives.
By using a food-grade expression system, this approach ensures that the D-allulose produced meets safety and regulatory standards for use in food.
Similarly, D-tagatose 3-epimerase from Sinorhizobium sp. achieved a conversion rate of 35% of D-fructose to D-allulose at 55 °C, retaining 85% of its activity after 24 h [ 45 ]. The robust performance of the enzyme under these conditions suggests its potential for industrial applications, especially where continuous operation is required.
Finally, the recombinant D-allulose-3-epimerase from Thermoclostridium caenicola showed a 38% conversion rate from D-fructose to D-allulose at 60 °C and retained 80% of its activity after 24 h [ 22 ]. Together, these enzymes show significant potential for integration into large-scale production systems, where their long-term consistent performance is essential to maintain process efficiency and reduce costs, opening the way for a wider application of D-allulose in various products.
Together, these studies highlight significant advances in enzyme characterization, thermostability improvements, and efficient biocatalytic applications that will advance the industrial production of D-allulose and other rare sugars.
By focusing on enzymes with high stability and activity under industrial conditions, these studies will lead to more efficient and cost-effective production processes, ultimately contributing to the availability of healthier allulose alternatives.
Table 1. Recent typical research on the properties of 3-epimerase.
2.1.3. Direct evolutionary study
Recent advances in the development of D-allulose-3-epimerase (DAEase) have improved its thermostability, catalytic efficiency, and acid resistance, which are crucial for industrial D-allulose production [ 22 ].
The use of directed evolution – a method of iterative mutation and selection – has demonstrated that single amino acid mutations can significantly improve the properties of an enzyme. When single mutations fail, neutral mutations often stabilize the protein, allowing for subsequent beneficial changes, or altering the “selected” functions to develop new capabilities.
This knowledge of directed evolution was applied to DAEase, leading to improved enzyme performance and more efficient, sustainable industrial production of low-calorie D-allulose [48].
Through directed evolution, mutants of Clostridium cellulolyticum H10 DAEase, such as D281G and C289R, showed significantly increased half-life at 65 °C, with the triple mutant, A107P/D281G/C289R, increasing the half-life by 58.85-fold and improving thermostability by 14.39 °C [ 43 ].
Similarly, computational tools identified beneficial mutations in the Thermoclostridium caenicola DAEase, resulting in the four-point Var3 mutant, which has increased rigidity and stability due to new hydrogen bonds and optimized electrostatic charge distribution [ 49 ].
A sequence and structure-based approach identified a highly thermostable TtDAE from Thermogutta terrifontis, with the M4 variant having a 5.12-fold increase in catalytic efficiency and a significantly higher melting temperature [ 48 ]. Proline amino acid substitutions in the Clostridium bolteae DAEase, particularly the Cb-51P/89P double mutant, led to a 2.32-fold increase in half-life at 55 °C [ 50 ].
Furthermore, engineering efforts for acid resistance resulted in the triple mutant M3 (I114R/K123E/H209R), which increased its activity by 3.36-fold and its acid resistance by 10.6-fold at pH 4.5, making it suitable for the production of functional juice [ 51 ].
Rational design of the Halanaerobium congolense DAEase resulted in the Y7H/C66L/I108A and Y7H/C66L/I108A/R156C/K260C mutants, the latter of which showed a half-life of 5.2 h at 70 °C and a melting temperature increase of 6.5 °C [ 52 ].
These studies highlight the synergy between computational predictions and experimental validation, revealing that enhancing enzyme stability and activity through targeted genetic modifications is key to industrial applications. The use of proline substitutions and disulfide bridges combined with B-factor analysis underscores the importance of understanding protein dynamics for efficient enzyme design [ 53 ].
The development of acid-resistant variants further expands the application of DAEase in acidic environments, demonstrating the versatility and potential of the enzyme.
Overall, these research efforts address current limitations of DAEase and set a precedent for future studies aimed at further optimizing biocatalysts, paving the way in biotechnology and beyond.
2.1.4. Synthetic biology method
Traditional whole-cell catalysis typically employs modified Corynebacterium glutamicum or Bacillus subtilis, and new metabolic pathways are continually being explored [8].
Recent research on the involvement of ATP in metabolic catalysis is noteworthy, and could bring significant changes to synthetic biology methods.
Synthetic biology methods can increase the conversion rate of allulose and upset the balance between fructose and allulose. The production of D-allulose by synthetic biology has gained significant interest due to its health benefits and economic value. This review compares three prominent methods: phosphorylation-driven production, thermodynamic-driven production, and the use of metabolically modified Escherichia coli, highlighting their integrated pathways and efficiencies [ 34 ].
Phosphorylation-driven production is characterized by its coupling with the ATP regeneration system. In this method, D-glucose is converted to D-allulose through a series of enzymatic steps. Key enzymes include hexokinase, which phosphorylates D-glucose to glucose-6-phosphate, and glucose-6-phosphate isomerase, which converts it to fructose-6-phosphate. Fructose-6-phosphate is further converted to allulose-6-phosphate by D-tagatose-3-epimerase, which is finally dephosphorylated to D-allulose. The ATP regeneration system also involves creatine kinase, which helps maintain high ATP levels, increasing the efficiency and yield of the production process [ 54 ].
A highly efficient pathway for the synthesis of D-allulose from D-fructose uses D-psicose epimerase and L-rhamnulose kinase, which are integrated with an ATP regeneration system via polyphosphate kinase (Figure 2) [55].
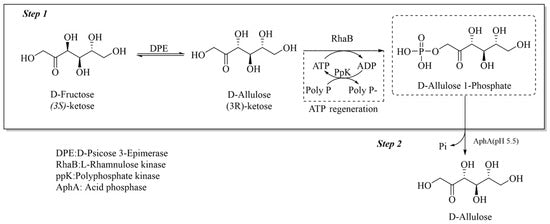
This method achieves an impressive 99% conversion rate by optimizing key reaction conditions, significantly reducing ATP consumption to 10% of the theoretical amount, and employing a batch dosing regimen to mitigate polyphosphate inhibition.
Traditionally, boric acid has been used to promote the D-allulose synthesis reaction through complexes formed with fructose, thereby shifting the equilibrium towards allulose production ( Figure 3 ).
However, while boric acid significantly increases the conversion yield by exploiting its high binding affinity for D-allulose, it poses environmental and health risks due to its toxicity and difficulty in removing it from the final product.
In contrast, the enzymatic approach exploits the natural catalytic efficiency of enzymes and the ATP regeneration cycle to sustainably promote the reaction without harmful additives.
This development highlights the potential of metabolic engineering to enhance enzyme efficiency and economy in biochemical production. Successful integration of ATP regeneration not only reduces costs but also eliminates the need for boric acid, making the process more environmentally friendly [56].

Figure 2. Conversion of D-fructose to D-allulose by phosphorylation method.
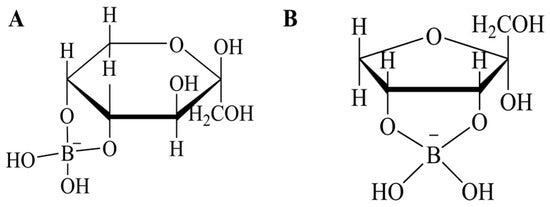
Figure 3. Chemical structures of sugar-borate complexes. ( A ) Fructose as β-d-fructopyranose cis-C-4,5 diol borate. ( B ) Psicose as α-d-furanopsicose cis-C-3,4 diol borate
3. Summary
Technological developments in allulose production focus not only on improving efficiency but also on addressing environmental and economic concerns.
Sustainable production methods, such as the use of renewable raw materials and green chemistry principles, are integrated into production processes. For example, the use of agricultural by-products as substrates for microbial fermentation reduces waste and adds value to otherwise low-value materials.
Economic analyses of these advanced production methods have shown that optimizing enzyme performance and using integrated production systems can significantly reduce the cost of allulose production.
These cost reductions are critical to making allulose a competitive alternative to traditional sweeteners on the market.
The rapid development of enzymatic conversion, synthetic biology, and integrated production systems is revolutionizing allulose production.
These technologies not only increase the efficiency and scalability of allulose production, but are also in line with global trends towards sustainable and economically viable manufacturing processes.
Future research should continue to focus on improving enzyme stability and activity through advanced protein design techniques.
In addition, the discovery of new microbial hosts and the optimization of their metabolic pathways can further improve microbial production systems. The integration of continuous production systems and the development of more efficient downstream processing techniques will be key to the commercial viability of allulose.
Collaboration between industry and academia is essential to turn these technological developments into practical applications.
By leveraging the expertise and resources of both sectors, it will be possible to overcome the challenges that remain in allulose production and fully exploit its potential as a healthy and sustainable sugar alternative.
4. Conclusions
The global trend in allulose production is characterized by a shift towards sustainable and efficient bio-based methods.
Key players are investing in advanced technologies to optimize production processes, and China is making a significant contribution through its investments in R&D and technological developments.
Collaboration between industry and academia is essential to overcome production challenges and meet the growing global demand for allulose.
Recent regulatory approvals, particularly by the FDA and the expected approval by EFSA, further increase the market potential of allulose, indicating a bright future for this innovative sugar substitute.
In addition, the recent approval by the Chinese National Health Commission significantly increases the domestic market potential of allulose, supporting its integration into a wide range of foods and in line with global health trends.

